One of the earliest decisions that an embryo faces is assignment of coordinates along which the body plan will develop. The dorsal/ventral (D/V) axis runs from the back through the belly of the animal, and in flies, fish and frogs there is remarkable conservation in the molecular mechanism that specifies this axis. In Drosophila, maternal factors guide the initial subdivision of the embryo along the D/V axis into the top and bottom halves. The top half, which will become the dorsal side of the embryo, is comprised of two tissues; the dorsal ectoderm that gives rise to portions of the skin, and the amnioserosawhich guides the movements of gastrulation. Mutational analysis has identified at least five zygotic genes that guide the dorsal sub-division and we have been actively investigating the biochemical mechanism behind this process.
The key gene products that patterns the dorsal half of the embryo is coded for by the decapentaplegic (dpp) and screw loci. Both Dpp and Scw are related to the bone morphogenetic proteins (BMPs) of vertebrates, which in turn are members of a very large group of secreted factors known as the TGF-ß superfamily of signaling molecules. Members of this family control a wide range of developmental and physiological processes in higher eukaryotes.
One of the more intriguing properties of certain TGF-ß-type proteins is their ability to act as morphogens; molecules that instruct cells to adopt specific fates in a concentration dependent manner. The Drosophila Dpp protein has morphogen properties since, in the embryo, different levels of Dpp distinguish amnioserosa from dorsal ectoderm. How is the gradient of Dpp concentration generated in dorsal tissue and how do the cells read the gradient to produce the appropriate response? We have found that gradient formation is controlled at the extra cellular level by interaction of Dpp and Scw with several other secreted factors that include the products of the short gastrulation (sog), twisted gastrulation (tsg), and tolloid (tld). The combined action of Sog, Tsg and Tld is to spatially localize a heterodimer of Dpp/Scw in a tight distribution centered about the dorsal midline, leading to amnioserosa development while the distributions of the Dpp/Dpp and Scw/Scw homodimers remain broad because they have low affinity for the Sog and Tsg proteins. The different ligand spatial profiles create a bi-phasic signal where amnioserosa precursors receive high levels of signal from the more potent Dpp/Scw heterodimer while dorsal ectoderm cells are specified by the low levels of signal elicited by the Dpp/Dpp and Scw/Scw homodimers.
More recently we have developed a reaction-diffuse model for D/V patterning and are using computational methods to explore the properties of this patterning system. For example, one important aspect of biological processes is that they need to be fairly plastic so that they function properly despite differences in the genetic makeup and environmental conditions under which the organism develops. Such systems are termed “robust” and for the D/V patterning process, we wished to determine the source of robustness. In collaboration with Dr. Hans Othmer from the Mathematics Department at the University of Minnesota, we have analyzed the behavior of theD/V patterning system with respect to changes in gene dose. What we have discovered is that Nature has employed heterodimeric molecules at each step of the signaling cascade and these heterodimers help buffer the patterning system to changes in the concentration of the individual monomeric components. Thus, a 2-fold change in monomer input results in much less change in the effective concentration of the heterodimer. We think this may be one reason why heterodimers are employed by many biological processes. We are further refining the computational model using 3D approximations to examine how the system integrates signal in both time and space. Our preliminary data suggests that the classic morphogen view does not hold in this case. That is, cell fate is not specified as a result of cells reading a steady state morphogen gradient. Instead, cells see a changing gradient over time, but are able to assume distinct fates because the integrate the signal using a positive feedback mechanism.
Click on the image below for a larger version
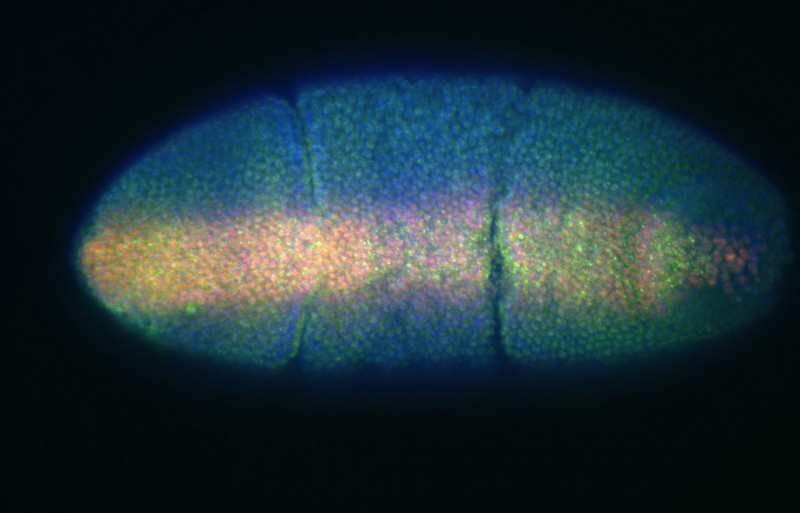
An early Drosophila embryo showing Dpp/Scw espression on the dorsal side (green) and Mad (red)
(M. O'Connor)